Arrhythmogenic Vulnerability of Reentrant Pathways in Post-Infarct Ventricular Tachycardia Assessed by Advanced Computational Modelling
Pranav Bhagirath MD PhD, Fernando O. Campos PhD, Pieter Postema MD PhD, Michiel J.B. Kemme MD PhD, Arthur A.M. Wilde MD PhD, Anton J. Prassl PhD, Aurel Neic PhD, Christopher A. Rinaldi MD PhD, Marco J.W. Götte MD PhD, Gernot Plank PhD, Martin J. Bishop PhD
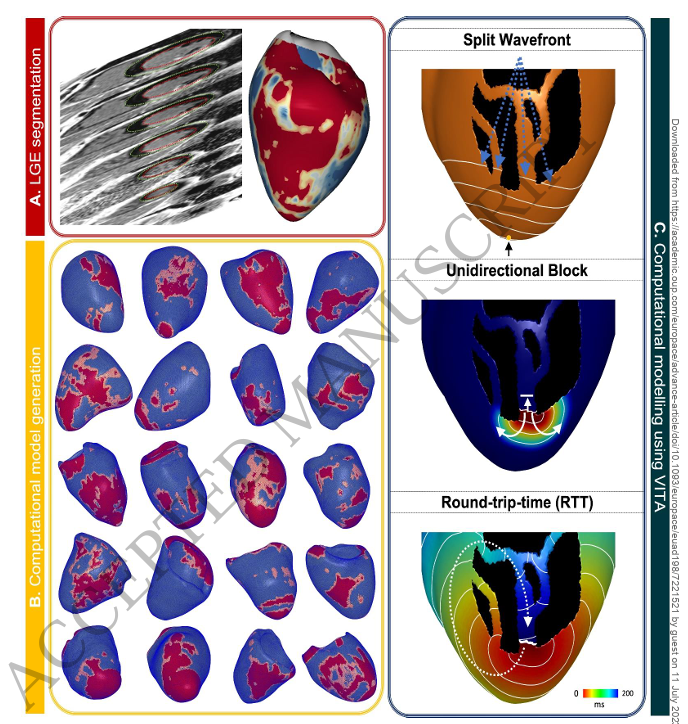
Background: Substrate assessment of scar-mediated ventricular tachycardia (VT) is frequently performed using late gadolinium enhancement (LGE) images. Although this provides structural information about critical pathways through the scar, assessing the vulnerability of these pathways for sustaining VT is not possible with imaging alone. This study evaluated the performance of a novel automated reentrant pathway finding algorithm to non-invasively predict VT circuit and inducibility.
Methods: 20 post-infarct VT-ablation patients were included for retrospective analysis. Commercially available software (ADAS3D LV) was used to generate scar maps from 2D-LGE images using the default 40-60 pixel-signal-intensity (PSI) threshold. In addition, algorithm sensitivity for altered thresholds was explored using PSI 45-55, 35-65 and 30-70. Simulations were performed on the Virtual Induction and Treatment of Arrhythmias (VITA) framework to identify potential sites of block and assess their vulnerability depending on the automatically-computed round-trip-time (RTT). Metrics, indicative of substrate complexity, were correlated with VT-recurrence during follow-up.
Results: 21 Total VTs (85±43 vs. 42±27) and unique VTs (9±4 vs. 5±4) were significantly higher in 22 patients with- compared to patients without recurrence, and were predictive of recurrence with AUC of .820 and .770, respectively. VITA was robust to scar threshold variations with no significant impact on total and unique VTs, and mean RTT between the four models. Simulation metrics derived from PSI 45-55 model had the highest number of parameters predictive for post-ablation VT-recurrence.
Conclusion: Advanced computational metrics can non-invasively and robustly assess VT substrate complexity, which may aid personalized clinical planning and decision making in treatment of post-infarction VT.